Pathogenesis of Irreversible Cell Injury: From Damage to Cell Death
Medi Study Go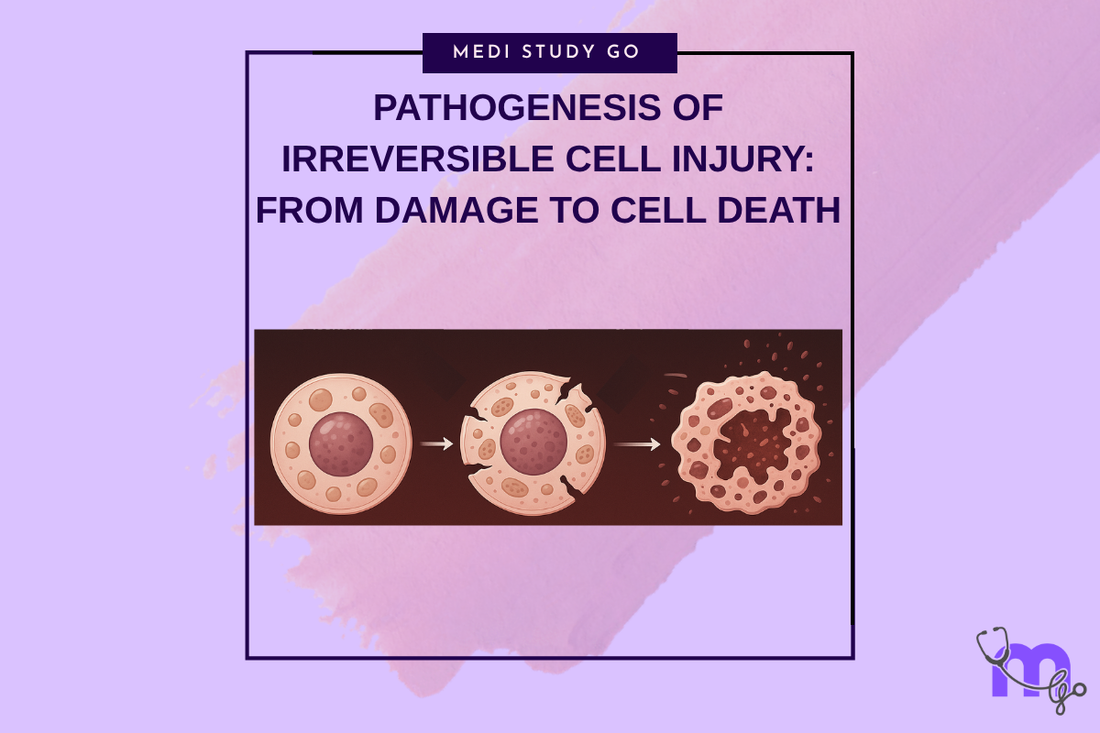
Related Resources
Cell Injury and Adaptation: The Foundation of Pathology
Morphology of Reversible Cell Injury: Key Features and Clinical Examples
Pathogenesis of Reversible Cell Injury: Mechanisms and Outcomes
Necrosis: Types, Mechanisms, and Clinical Significance
Apoptosis: Programmed Cell Death in Health and Disease
Pathological Calcification: Dystrophic vs. Metastatic
Cellular Adaptation: Types, Mechanisms, and Clinical Relevance
Intracellular Accumulations: Lipids, Proteins, Pigments, and More
Cell Injury in Clinical Practice in Dentistry
Key Takeaways
- Irreversible cell injury occurs when cellular damage exceeds repair capacity, leading to inevitable cell death through necrosis or apoptosis
- Membrane damage represents the critical point of no return, causing loss of cellular compartmentalization and uncontrolled ion flux
- Free radical generation and lipid peroxidation create cascading damage that overwhelms cellular antioxidant defenses and repair mechanisms
- DNA damage and protein fragmentation disrupt essential cellular functions beyond the point of recovery
- Recognition of irreversible injury markers enables appropriate treatment decisions and prevents futile therapeutic interventions
Introduction

The pathogenesis of irreversible cell injury represents a critical transition point where cellular damage becomes so extensive that recovery is impossible, leading inevitably to cell death through either necrotic or apoptotic pathways. Understanding this pathogenesis is essential for dental professionals who must distinguish between conditions that can be successfully treated and those requiring acceptance of tissue loss and appropriate management of consequences.
Irreversible cell injury develops when the magnitude and duration of injurious stimuli exceed the cellular capacity for adaptation and repair. This transition from reversible to irreversible injury occurs at specific threshold points that represent the point of no return, beyond which therapeutic interventions cannot restore cellular viability and function.
The mechanisms underlying irreversible cell injury involve cascading processes that amplify initial damage and create multiple points of cellular dysfunction simultaneously. Unlike reversible injury where single mechanism disruption can be corrected, irreversible injury involves multiple simultaneous failures that overwhelm cellular repair capacity and lead to systematic cellular breakdown.
In dental practice, irreversible cell injury is encountered in conditions such as pulpal necrosis, advanced periodontal destruction, tissue necrosis following trauma or ischemia, and complications from dental procedures. Understanding the pathogenesis enables practitioners to recognize when tissue preservation is no longer possible and appropriate management strategies must focus on preventing complications and optimizing healing.
Contemporary research in cell death mechanisms continues to refine our understanding of the molecular events that determine irreversibility, with particular focus on identifying early markers that predict progression to cell death. These advances provide opportunities for more precise prognostic assessment and earlier intervention to prevent progression from reversible to irreversible injury.
Table of Contents
Critical Membrane Damage and Point of No Return What Triggers Free Radical Generation and Oxidative Damage? DNA Damage and Nuclear Changes in Cell Death How Do Cellular Organelles Fail in Irreversible Injury? Molecular Pathways Leading to Cell Death
Critical Membrane Damage and Point of No Return
Membrane Integrity Loss
Membrane damage represents the critical determinant of irreversible cell injury, as loss of membrane integrity results in uncontrolled movement of ions and molecules across cellular boundaries, leading to disruption of essential cellular compartmentalization. This damage affects both the cell membrane and intracellular organelle membranes, creating multiple points of cellular dysfunction that cannot be repaired.
The progression from reversible membrane dysfunction to irreversible membrane damage involves several key events including extensive lipid peroxidation, protein denaturation, and structural membrane disruption that exceeds the cellular capacity for repair. These changes result in loss of membrane selectivity and formation of membrane pores that allow uncontrolled ion flux.
Critical membrane damage is characterized by the inability to restore normal ion gradients even when cellular energy production is restored. This irreversible loss of membrane function creates a self-perpetuating cycle of cellular dysfunction that leads inevitably to cell death regardless of therapeutic interventions.
Lysosomal Membrane Disruption
Lysosomal membrane damage represents a particularly significant aspect of irreversible cell injury, as disruption of these organelles releases digestive enzymes into the cytoplasm, leading to autodigestion of cellular components. This process creates extensive cellular damage that cannot be repaired and accelerates progression to cell death.
The release of lysosomal enzymes including proteases, nucleases, and phospholipases creates widespread cellular damage affecting proteins, nucleic acids, and membrane lipids simultaneously. This multi-target damage overwhelms cellular repair mechanisms and creates the characteristic morphological changes associated with irreversible injury.
Lysosomal membrane stabilization represents a potential therapeutic target for preventing progression to irreversible injury, though once extensive lysosomal damage has occurred, this intervention is no longer effective in preventing cell death.
Cellular Membrane vs. Organelle Damage
The distinction between cell membrane and organelle membrane damage is crucial for understanding the progression of irreversible injury. While cell membrane damage allows loss of cellular contents and uncontrolled influx of extracellular substances, organelle membrane damage disrupts internal cellular organization and function.
Mitochondrial membrane damage is particularly significant as it disrupts cellular energy production beyond the point of recovery, while endoplasmic reticulum membrane damage disrupts protein synthesis and calcium homeostasis. The combination of multiple organelle membrane failures creates the comprehensive cellular dysfunction characteristic of irreversible injury.
The timing and sequence of membrane damage events influence the specific pathways of cell death, with some patterns leading to necrotic death while others trigger apoptotic pathways. Understanding these patterns helps predict cellular responses and plan appropriate therapeutic interventions.
What Triggers Free Radical Generation and Oxidative Damage?
Free radical generation represents a central mechanism in the pathogenesis of irreversible cell injury, creating cascading damage that overwhelms cellular antioxidant defenses and leads to extensive cellular destruction. Understanding these mechanisms is crucial for recognizing conditions that predispose to irreversible injury and implementing preventive strategies.
Sources of Free Radical Production
Mitochondrial dysfunction during cellular injury leads to increased production of reactive oxygen species including superoxide anion, hydrogen peroxide, and hydroxyl radicals that can damage multiple cellular components simultaneously. This production occurs when normal electron transport is disrupted, leading to electron leakage and formation of reactive species.
Inflammatory cell activation represents another significant source of free radical generation, particularly relevant in dental infections and inflammatory conditions. Neutrophils and macrophages produce large quantities of reactive oxygen and nitrogen species as part of their antimicrobial function, but these species can also damage surrounding tissues.
Reperfusion injury following ischemia creates a particularly intense burst of free radical generation as oxygen is reintroduced to tissues with compromised antioxidant defenses. This mechanism is relevant in dental practice during restoration of blood flow following trauma or surgical procedures.
Lipid Peroxidation and Membrane Damage
Lipid peroxidation represents one of the most significant consequences of free radical generation, leading to progressive membrane damage that can progress from reversible to irreversible injury. This process involves attack of reactive oxygen species on membrane phospholipids, creating lipid radicals that propagate damage through chain reactions.
The products of lipid peroxidation including aldehydes and other reactive compounds can damage proteins and nucleic acids, creating widespread cellular damage beyond the initial membrane effects. These secondary damage products contribute to the progressive nature of oxidative injury and the difficulty of reversing established damage.
Antioxidant depletion occurs as cellular antioxidant systems become overwhelmed by free radical production, creating conditions where oxidative damage accelerates and becomes self-perpetuating. Understanding this process emphasizes the importance of early intervention before antioxidant reserves are exhausted.
Protein and DNA Oxidative Damage
Protein oxidation leads to enzyme inactivation, structural protein damage, and formation of protein aggregates that can disrupt cellular function. This damage affects both enzymatic and structural proteins, creating widespread cellular dysfunction that contributes to irreversible injury development.
DNA oxidative damage can result in strand breaks, base modifications, and chromosomal aberrations that compromise cellular genetic integrity. While cells have repair mechanisms for DNA damage, extensive oxidative DNA damage can overwhelm these systems and contribute to cell death.
The relationship between oxidative damage and other injury mechanisms creates amplifying effects where initial damage leads to increased free radical production, which causes additional damage in a self-perpetuating cycle that characterizes irreversible cell injury.
DNA Damage and Nuclear Changes in Cell Death
Nuclear Morphological Changes
Nuclear changes in irreversible cell injury follow characteristic patterns that reflect the underlying mechanisms of DNA damage and cellular death pathways. Pyknosis involves nuclear shrinkage and chromatin condensation that occurs as DNA becomes fragmented and cellular repair mechanisms fail.
Karyorrhexis represents nuclear fragmentation where the condensed nucleus breaks into fragments that may be dispersed throughout the cytoplasm. This change indicates extensive DNA damage and represents an irreversible step in the cell death process.
Karyolysis involves nuclear dissolution and disappearance of nuclear staining that occurs as DNA is completely degraded by activated nucleases. This represents the final stage of nuclear destruction in cell death and indicates complete loss of genetic material.
DNA Fragmentation Mechanisms
Endonuclease activation represents a key mechanism in DNA fragmentation during irreversible cell injury. These enzymes become activated by increased intracellular calcium and other cellular stress signals, leading to systematic DNA breakdown that cannot be repaired.
The pattern of DNA fragmentation differs between necrotic and apoptotic cell death, with necrosis showing random DNA breaks while apoptosis demonstrates characteristic DNA laddering patterns. Understanding these patterns helps distinguish between different cell death mechanisms.
DNA repair mechanism failure occurs when the extent of DNA damage exceeds cellular repair capacity or when cellular energy depletion prevents normal repair processes. This failure represents a critical point where cells cannot maintain genetic integrity and progress to inevitable death.
Chromatin Structure Disruption
Chromatin clumping and condensation occur early in irreversible injury and reflect disrupted nuclear organization and altered DNA-protein interactions. These changes affect gene expression and cellular function while indicating progression toward cell death.
Histone modifications during cell death influence chromatin structure and accessibility, affecting the cellular response to injury and the specific pathways of cell death activation. Understanding these modifications provides insight into the molecular mechanisms controlling cell death processes.
Nuclear envelope breakdown represents a late change in cell death that allows mixing of nuclear and cytoplasmic contents, leading to further cellular disruption and indicating irreversible progression toward cell death.
How Do Cellular Organelles Fail in Irreversible Injury?
Mitochondrial Destruction
Mitochondrial failure in irreversible cell injury involves multiple simultaneous dysfunctions including loss of membrane potential, cessation of ATP production, and release of pro-apoptotic factors that can trigger cell death pathways. The appearance of large amorphous densities within mitochondria indicates irreversible structural damage.
Mitochondrial swelling progresses beyond the reversible changes seen in early injury to structural disruption that cannot be repaired. This includes cristae destruction, matrix density changes, and membrane rupture that eliminate the organelle's capacity for energy production.
Release of cytochrome c and other mitochondrial proteins into the cytoplasm triggers apoptotic pathways and represents a point of no return in cell death processes. Understanding this mechanism helps distinguish between reversible mitochondrial dysfunction and irreversible mitochondrial destruction.
Endoplasmic Reticulum Collapse
Endoplasmic reticulum fragmentation and loss of organization occur in irreversible injury as membrane integrity is lost and normal ER structure cannot be maintained. This disrupts protein synthesis, calcium homeostasis, and other essential ER functions.
Ribosomal detachment becomes irreversible when ER membrane damage is extensive, leading to complete cessation of protein synthesis that cannot be restored. This represents loss of the cellular capacity for repair and recovery.
ER stress responses become overwhelmed in irreversible injury, leading to activation of cell death pathways when cellular protective mechanisms cannot cope with the extent of ER dysfunction.
Lysosomal and Cytoskeletal Breakdown
Lysosomal enzyme release creates widespread cellular autodigestion that represents one of the most destructive aspects of irreversible cell injury. These enzymes digest cellular proteins, nucleic acids, and other components, creating the characteristic morphological changes of cell death.
Cytoskeletal dissolution leads to loss of cellular shape and organization, contributing to the morphological changes that characterize dying cells. This process affects cellular function and contributes to the inability to maintain normal cellular structure.
Formation of myelin figures from membrane breakdown products represents a characteristic feature of irreversible injury that indicates extensive membrane damage and cellular destruction.
Molecular Pathways Leading to Cell Death
Necrotic Pathway Activation
Necrotic cell death occurs through uncontrolled cellular breakdown characterized by cellular swelling, membrane rupture, and inflammatory response. This pathway is triggered by severe cellular injury that overwhelms adaptive mechanisms and leads to cellular destruction.
The molecular mechanisms of necrosis include ATP depletion, ion homeostasis failure, and membrane damage that creates uncontrolled cellular breakdown. Unlike apoptosis, necrosis does not involve specific signaling pathways but represents cellular failure due to overwhelming injury.
Inflammatory responses to necrotic cell death can cause additional tissue damage and represent an important consideration in clinical management of conditions involving tissue necrosis.
Apoptotic Pathway Triggering
Apoptotic cell death represents a controlled process triggered by specific cellular signals including DNA damage, growth factor withdrawal, and cellular stress that exceeds adaptive capacity. This pathway involves systematic cellular dismantling while maintaining membrane integrity.
Caspase activation represents the central mechanism in apoptotic cell death, with these proteolytic enzymes systematically dismantling cellular components in an organized fashion. Understanding caspase pathways provides insight into therapeutic approaches for modulating cell death.
The distinction between apoptotic and necrotic pathways influences tissue responses and clinical management, with apoptotic death generally causing less inflammatory response and tissue damage.
Therapeutic Implications and Intervention Points
Recognition of irreversible injury mechanisms enables appropriate treatment decisions that focus on managing consequences rather than attempting futile cellular rescue. This includes preventing secondary complications and optimizing healing of surrounding tissues.
Understanding cell death pathways guides selection of appropriate therapeutic approaches, with some interventions potentially influencing the specific pathway of cell death and its consequences for surrounding tissues.
The timing of therapeutic interventions relative to the progression of irreversible injury influences outcomes, emphasizing the importance of early recognition and appropriate intervention strategies.
Conclusion
Understanding the pathogenesis of irreversible cell injury provides critical knowledge for recognizing when cellular damage has progressed beyond the point of recovery and appropriate treatment strategies must focus on managing consequences rather than attempting cellular rescue. The complex mechanisms involving membrane damage, free radical generation, DNA fragmentation, and organelle failure create cascading processes that overwhelm cellular repair capacity.
Recognition of the critical transition points from reversible to irreversible injury enables more appropriate treatment decisions and prevents futile therapeutic interventions while optimizing management of tissue loss and complications. The molecular mechanisms underlying irreversible injury continue to provide insights into potential therapeutic targets for preventing progression from reversible injury.
Clinical applications of understanding irreversible injury pathogenesis include improved prognostic assessment, more appropriate treatment planning, and better patient counseling regarding treatment outcomes and expectations. Contemporary research continues to identify new aspects of cell death mechanisms that may provide therapeutic opportunities.
The integration of molecular understanding with clinical practice represents the foundation for advancing dental medicine toward more precise diagnostic and therapeutic approaches that optimize patient outcomes while avoiding unnecessary interventions when tissue damage has progressed beyond recovery.