Mechanism of Local Anesthetics: Sodium Channel Blockade and Nerve Conduction
Medi Study Go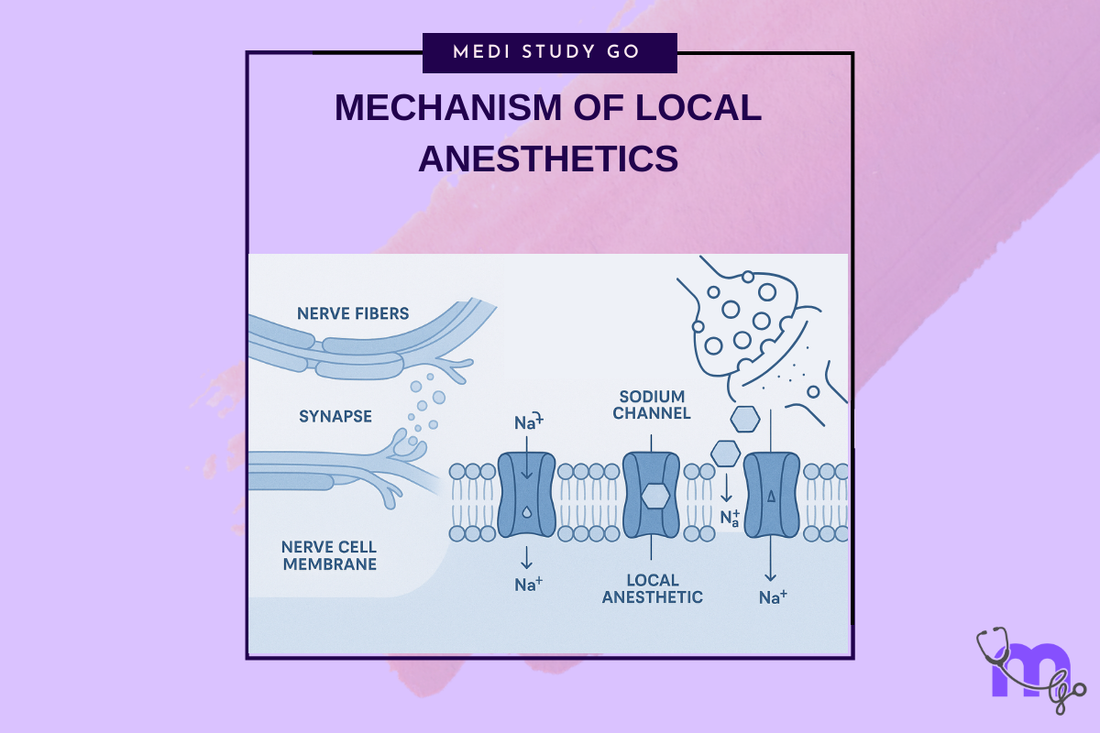
Related Resources
Local and General Anesthesia in Dentistry: Mechanisms, Techniques, and Clinical Applications for Dental Students Theories of Pain and Gate Control Theory: Relevance to Dental Anesthesia Classification of Local Anesthetics: Amides vs. Esters and Clinical Selection Criteria Inferior Alveolar Nerve Block: Step-by-Step Technique and Common Errors Complications of Local Anesthesia: Toxicity, Paresthesia, and Management Protocols Dose Calculation and Contraindications for Local Anesthetics in High-Risk Patients Gow-Gates and Closed-Mouth Nerve Blocks: Advanced Techniques for Mandibular Anesthesia Nitrous Oxide in Dentistry: Pharmacology, Sedation Stages, and Safety Protocols Premedication Strategies: Managing Dental Anxiety and Adrenal Insufficiency General Anesthesia in Dental Surgery: Indications, Stages, and Emergency Preparedness Eutectic Mixtures and Topical Anesthetics: Enhancing Patient Comfort in Pediatric Dentistry
Key Takeaways
- Local anesthetics block nerve conduction by binding to specific receptors within voltage-gated sodium channels, preventing depolarization
- The unionized form of the drug crosses the nerve membrane, while the ionized form binds to the receptor site inside the sodium channel
- pH significantly affects local anesthetic efficacy - lower pH in infected tissues reduces the proportion of active unionized drug
- Different theories explain local anesthetic action, with the specific receptor theory being most widely accepted
- Factors like protein binding, lipid solubility, and pKa determine onset, potency, and duration of local anesthetics
Local anesthetics represent one of the most significant advances in dentistry, enabling painless procedures that were once dreaded by patients. Understanding the precise mechanism by which these drugs block nerve conduction is essential for dental professionals to optimize their use and troubleshoot when anesthesia fails. This comprehensive exploration delves into the molecular interactions between local anesthetics and nerve tissues.
Table of Contents
- Neurophysiology of Nerve Conduction
- The Sodium Channel: Structure and Function
- Molecular Mechanism of Local Anesthetic Action
- Theories of Local Anesthetic Action
- Factors Affecting Local Anesthetic Efficacy
Neurophysiology of Nerve Conduction

The Resting Membrane Potential
Nerve cells maintain a resting membrane potential of approximately -70 to -90 millivolts, with the interior negative relative to the exterior. This potential difference results from:
Selective membrane permeability - The neuronal membrane is selectively permeable to different ions, with greater permeability to potassium than sodium at rest.
Sodium-potassium pump - This active transport mechanism moves three sodium ions out for every two potassium ions moved in, contributing to the negative internal charge.
Protein anions - Large, negatively charged proteins trapped within the cell contribute to the overall negative charge.
Action Potential Generation
When a nerve is stimulated, a cascade of events leads to signal propagation:
- Threshold stimulation causes initial depolarization
- Voltage-gated sodium channels open rapidly
- Sodium influx causes rapid depolarization to +40 mV
- Sodium channel inactivation occurs at peak depolarization
- Potassium channel opening allows K+ efflux
- Repolarization returns membrane to resting potential
- Hyperpolarization briefly occurs before full recovery
This sequence propagates along the nerve fiber, transmitting the signal without decrement.
Conduction in Myelinated vs. Unmyelinated Fibers
Myelinated fibers conduct via saltatory conduction, where action potentials "jump" between nodes of Ranvier. This mechanism:
- Increases conduction velocity dramatically
- Conserves energy by limiting depolarization to nodes
- Makes these fibers more susceptible to local anesthetic block at nodes
Unmyelinated fibers conduct continuously along the entire axon length:
- Slower conduction velocity
- Require local anesthetic contact along greater nerve length
- Generally more resistant to anesthetic block
The Sodium Channel: Structure and Function
Molecular Architecture
The voltage-gated sodium channel is a complex protein structure consisting of:
Alpha subunit - The main pore-forming unit containing:
- Four homologous domains (I-IV)
- Six transmembrane segments per domain (S1-S6)
- Voltage sensor in S4 segment
- Pore region between S5 and S6
Beta subunits - Auxiliary proteins that:
- Modulate channel kinetics
- Affect channel localization
- Influence local anesthetic binding
Functional States
Sodium channels exist in three primary conformational states:
Resting (closed) - Channel is closed but capable of activation
- Occurs at normal resting membrane potential
- Local anesthetics have low affinity for this state
Activated (open) - Channel opens allowing sodium influx
- Triggered by membrane depolarization
- Brief duration (1-2 milliseconds)
- Moderate local anesthetic affinity
Inactivated (closed) - Channel is closed and refractory
- Occurs following activation
- Highest affinity for local anesthetics
- Must return to resting state before reactivation
The Local Anesthetic Binding Site
Research has identified the local anesthetic binding site within the alpha subunit:
- Located in the S6 segment of domain IV
- Accessible from the intracellular side
- Contains specific amino acid residues critical for drug binding
- Overlaps with the channel's inactivation gate
Molecular Mechanism of Local Anesthetic Action
The Journey of a Local Anesthetic Molecule
The complex process by which local anesthetics block nerve conduction involves several steps:
- Injection and diffusion - The anesthetic solution spreads through tissues toward the target nerve
- Membrane penetration - The unionized (base) form crosses the lipophilic nerve membrane
- Ionization - Inside the nerve cell, the lower pH causes protonation, creating the active cationic form
- Receptor binding - The ionized form binds to specific receptors within the sodium channel
- Channel blockade - Bound anesthetic prevents sodium influx, blocking depolarization
- Use-dependent block - Repeated nerve stimulation enhances blockade by increasing access to binding sites
pH-Dependent Activity
The Henderson-Hasselbalch equation governs the ionization state:
pH = pKa + log([B]/[BH+])Where:
- pH = tissue pH
- pKa = dissociation constant of the anesthetic
- [B] = concentration of base (unionized) form
- [BH+] = concentration of ionized form
Clinical implications:
- Normal tissue pH (7.4) provides optimal balance
- Infected tissue (pH ~6.5) reduces unionized fraction
- Alkalinization of anesthetic solutions can improve efficacy
Calcium Displacement Theory
Local anesthetics may also displace calcium from membrane binding sites:
- Calcium stabilizes the nerve membrane
- Displacement increases membrane fluidity
- This may facilitate anesthetic penetration
- Contributes to membrane expansion theory
Theories of Local Anesthetic Action
Specific Receptor Theory
The most widely accepted mechanism proposes direct binding to sodium channels:
Evidence supporting this theory:
- Specific binding sites identified within channels
- Stereoselectivity of some local anesthetics
- Correlation between binding affinity and potency
- Use-dependent block phenomenon
Molecular interactions:
- Hydrophobic interactions with channel proteins
- Electrostatic attraction to negatively charged residues
- Hydrogen bonding with specific amino acids
- Steric fit within the binding pocket
Membrane Expansion Theory
This theory suggests local anesthetics disorder membrane lipids:
Proposed mechanisms:
- Integration into membrane bilayer
- Disruption of lipid packing
- Altered membrane fluidity
- Secondary effects on embedded proteins
Limitations:
- Doesn't explain stereoselectivity
- Poor correlation with clinical potency
- Concentrations needed exceed clinical use
Surface Charge Theory
Local anesthetics may alter membrane surface charge:
Mechanisms:
- Cationic drug binding to anionic membrane sites
- Alteration of electrical field across membrane
- Changed threshold for channel activation
- Modified channel gating kinetics
Acetylcholine Theory
Historical theory suggesting interference with acetylcholine:
Proposed mechanisms:
- Competition for cholinergic receptors
- Inhibition of acetylcholine synthesis
- Altered neurotransmitter release
Current status:
- Largely disproven for local anesthetic action
- May contribute to CNS effects at toxic doses
- Not relevant for peripheral nerve block
Factors Affecting Local Anesthetic Efficacy
Physicochemical Properties
Lipid solubility determines:
- Potency (more lipophilic = more potent)
- Ability to penetrate nerve membranes
- Distribution in fatty tissues
- Duration of action
Protein binding influences:
- Duration of action (higher binding = longer duration)
- Resistance to washout from tissues
- Plasma protein interactions
- Systemic toxicity potential
pKa affects:
- Onset time (lower pKa = faster onset)
- Proportion of unionized drug at tissue pH
- Ability to penetrate infected tissues
- Efficacy in different tissue environments
Tissue Factors
Vascularity impacts:
- Drug absorption rate
- Duration of local action
- Systemic toxicity risk
- Need for vasoconstrictors
Nerve fiber characteristics:
- Diameter (smaller fibers blocked first)
- Myelination (affects drug access)
- Firing frequency (use-dependent block)
- Position in nerve bundle (peripheral vs. core)
Tissue pH determines:
- Ionization state of anesthetic
- Membrane penetration ability
- Onset time and efficacy
- Failure in infected tissues
Pathological Conditions
Inflammation affects anesthesia through:
- Decreased tissue pH
- Increased vascularity
- Altered protein binding
- Changed drug distribution
Infection creates multiple challenges:
- Acidic environment reduces efficacy
- Increased tissue barriers
- Enhanced drug absorption
- Risk of spreading infection
Previous surgery or radiation:
- Scar tissue impedes diffusion
- Altered nerve anatomy
- Changed tissue pH
- Modified vascular patterns
Clinical Factors
Injection technique influences:
- Drug deposition accuracy
- Concentration at target site
- Risk of intravascular injection
- Patient comfort and anxiety
Volume and concentration:
- Total drug dose
- Spread through tissues
- Duration of effect
- Systemic absorption rate
Addition of vasoconstrictors:
- Prolongs duration
- Reduces systemic toxicity
- Increases block intensity
- May affect onset time
Individual Patient Factors
Genetic variations in:
- Sodium channel structure
- Drug metabolism enzymes
- Receptor sensitivity
- Pain perception pathways
Age-related changes:
- Altered nerve conduction
- Changed drug distribution
- Modified metabolism
- Increased sensitivity in elderly
Medical conditions affecting efficacy:
- Liver disease (amide metabolism)
- Cardiac conditions (epinephrine sensitivity)
- Neurological disorders
- Psychological factors
Understanding these mechanisms enables clinicians to:
- Select appropriate anesthetics for specific situations
- Troubleshoot when anesthesia fails
- Minimize complications
- Optimize patient comfort
The molecular mechanism of local anesthetic action represents a remarkable example of applied pharmacology. By specifically targeting sodium channels and preventing nerve conduction, these drugs have revolutionized dental practice. Continued research into these mechanisms promises even more effective and safer anesthetic agents in the future.