Classification of Local Anesthetics: Amides vs. Esters and Clinical Selection Criteria
Medi Study Go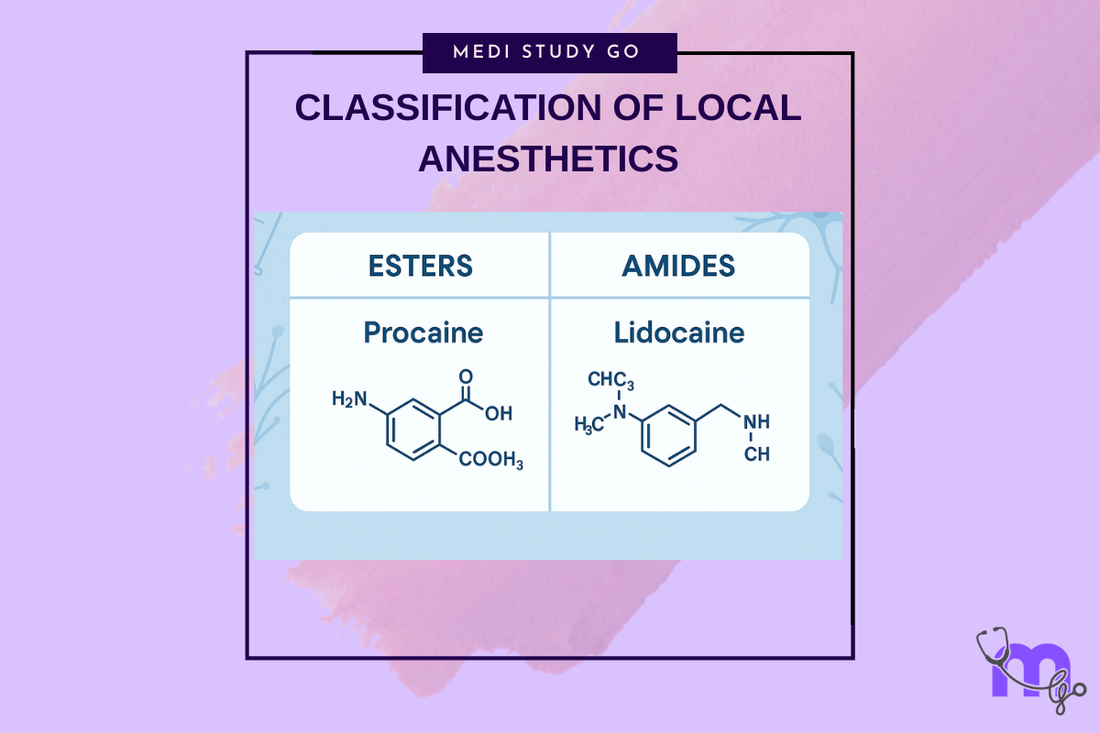
Related Resources
Local and General Anesthesia in Dentistry: Mechanisms, Techniques, and Clinical Applications for Dental Students Theories of Pain and Gate Control Theory: Relevance to Dental Anesthesia Mechanism of Local Anesthetics: Sodium Channel Blockade and Nerve Conduction Inferior Alveolar Nerve Block: Step-by-Step Technique and Common Errors Complications of Local Anesthesia: Toxicity, Paresthesia, and Management Protocols Dose Calculation and Contraindications for Local Anesthetics in High-Risk Patients Gow-Gates and Closed-Mouth Nerve Blocks: Advanced Techniques for Mandibular Anesthesia Nitrous Oxide in Dentistry: Pharmacology, Sedation Stages, and Safety Protocols Premedication Strategies: Managing Dental Anxiety and Adrenal Insufficiency General Anesthesia in Dental Surgery: Indications, Stages, and Emergency Preparedness Eutectic Mixtures and Topical Anesthetics: Enhancing Patient Comfort in Pediatric Dentistry
Key Takeaways
- Amide local anesthetics contain two "i"s in their name and are metabolized in the liver, while esters have one "i" and are broken down by plasma cholinesterase
- Amides have a significantly lower allergic potential (<1%) compared to esters, making them the preferred choice in modern dentistry
- Articaine is unique among amides, containing both amide and ester linkages, allowing dual metabolism pathways
- Clinical selection depends on factors including duration needed, patient medical history, allergic potential, and specific procedure requirements
- Cross-reactivity exists among esters but not between amides and esters, important for managing allergic patients
The classification of local anesthetics into amides and esters represents more than just chemical nomenclature - it fundamentally determines their pharmacokinetics, safety profiles, and clinical applications. Understanding these differences enables dental professionals to make informed choices that optimize patient care while minimizing risks.
Table of Contents
- Chemical Structure and Classification
- Metabolism and Pharmacokinetics
- Individual Drug Profiles
- Clinical Selection Criteria
- Special Considerations and Contraindications
Chemical Structure and Classification
Basic Structural Components
All local anesthetics share three essential structural components:
Lipophilic aromatic ring - Usually a benzene ring that:
- Provides fat solubility for membrane penetration
- Determines potency (more lipophilic = more potent)
- Influences duration of action
- Affects protein binding
Intermediate chain - Connects the aromatic ring to the amine group:
- Contains either an ester (-COO-) or amide (-NHCO-) linkage
- Determines the drug classification
- Influences metabolism pathway
- Affects stability and shelf life
Hydrophilic amino group - Usually a tertiary amine that:
- Accepts protons to form water-soluble salts
- Becomes ionized at physiological pH
- Essential for receptor binding
- Influences onset time through pKa
The Ester-Amide Dichotomy
Ester local anesthetics are characterized by:
- Ester linkage (-COO-) in the intermediate chain
- Generic names with one "i" (procaine, tetracaine, benzocaine)
- Metabolism by plasma pseudocholinesterase
- Production of para-aminobenzoic acid (PABA) metabolite
- Higher allergenic potential
Amide local anesthetics are distinguished by:
- Amide linkage (-NHCO-) in the intermediate chain
- Generic names with two "i"s (lidocaine, mepivacaine, prilocaine)
- Hepatic metabolism via cytochrome P450 enzymes
- More stable chemical structure
- Extremely low allergenic potential
Unique Structural Variations
Articaine - The hybrid molecule:
- Contains both amide and ester components
- Thiophene ring instead of benzene
- Dual metabolism pathways
- Enhanced lipid solubility
Prilocaine - The secondary amine:
- Only dental anesthetic with secondary amine
- Unique metabolic pathway
- Risk of methemoglobinemia
- Different pharmacokinetic profile
Metabolism and Pharmacokinetics
Ester Metabolism
Ester local anesthetics undergo hydrolysis by pseudocholinesterase:
Plasma metabolism occurs rapidly:
- Half-life measured in minutes
- Produces PABA and alcohol derivatives
- Rapid clearance reduces toxicity risk
- Affected by cholinesterase deficiency
Clinical implications:
- Shorter duration of action
- Lower systemic toxicity potential
- Higher allergic reaction risk
- Contraindicated with sulfonamides
Factors affecting ester metabolism:
- Genetic cholinesterase variants
- Liver disease (produces cholinesterase)
- Pregnancy (decreased enzyme levels)
- Concurrent medications
Amide Metabolism
Amide local anesthetics undergo hepatic biotransformation:
Phase I metabolism involves:
- N-dealkylation
- Hydroxylation
- Cytochrome P450 enzyme systems
- Production of active metabolites
Phase II metabolism includes:
- Conjugation reactions
- Glucuronidation
- Formation of water-soluble metabolites
- Renal excretion
Clinical implications:
- Longer half-life (90-120 minutes)
- Accumulation risk with repeated doses
- Caution in hepatic dysfunction
- Drug interaction potential
Articaine: A Special Case
Articaine combines metabolism pathways:
Plasma hydrolysis (90%):
- Ester side chain cleaved by esterases
- Rapid initial metabolism
- Reduces hepatic burden
Hepatic metabolism (10%):
- Remaining drug processed in liver
- Shorter half-life than other amides
- Reduced accumulation risk
Individual Drug Profiles
Amide Local Anesthetics
Lidocaine (Xylocaine)
- Gold standard in dentistry
- pKa: 7.9 (moderate onset)
- Duration: 90-150 minutes with vasoconstrictor
- Maximum dose: 7 mg/kg with epinephrine
- Versatile use: infiltration and blocks
Mepivacaine (Carbocaine)
- Mild vasoconstrictive properties
- pKa: 7.6 (rapid onset)
- Duration: 20-40 minutes plain, 60-90 with vasoconstrictor
- Useful for short procedures
- Good choice for cardiac patients
Articaine (Septocaine)
- Superior bone penetration
- pKa: 7.8
- Duration: 60-90 minutes
- Maximum dose: 7 mg/kg
- Excellent for infiltration anesthesia
Prilocaine (Citanest)
- Least vasodilatory effect
- pKa: 7.9
- Duration: 60-120 minutes
- Risk of methemoglobinemia
- Good for longer procedures
Bupivacaine (Marcaine)
- Long-acting anesthetic
- pKa: 8.1 (slow onset)
- Duration: 240-540 minutes
- High cardiotoxicity risk
- Reserved for post-operative pain
Ester Local Anesthetics
Procaine (Novocain)
- Historical significance
- pKa: 8.9 (very slow onset)
- Duration: 15-30 minutes
- Poor tissue penetration
- Rarely used in modern dentistry
Tetracaine
- Primarily topical use
- pKa: 8.5
- Long duration topically
- High toxicity potential
- Excellent mucous membrane penetration
Benzocaine
- Topical use only
- Unique structure (lacks terminal amine)
- Rapid onset for topical anesthesia
- Risk of methemoglobinemia
- Available in various concentrations
Clinical Selection Criteria
Procedure-Based Selection
Short procedures (<30 minutes):
- Plain mepivacaine
- 2% lidocaine with epinephrine
- Consider patient turnover
Intermediate procedures (30-60 minutes):
- Lidocaine with epinephrine
- Articaine with epinephrine
- Prilocaine with or without vasoconstrictor
Long procedures (>60 minutes):
- Bupivacaine for post-operative control
- Combination techniques
- Consider re-injection timing
Patient-Based Selection
Healthy adults:
- Standard amide anesthetics
- Epinephrine-containing solutions
- Full range of options available
Pediatric patients:
- Lower maximum doses (mg/kg basis)
- Shorter-acting agents preferred
- Avoid prilocaine in young children
Elderly patients:
- Reduced doses may be needed
- Slower metabolism consideration
- Careful with vasoconstrictors
Pregnant patients:
- Category B drugs preferred (lidocaine)
- Avoid elective procedures in first trimester
- Minimize total drug exposure
Medical Condition Considerations
Cardiovascular disease:
- Limit epinephrine concentration
- Consider plain solutions
- Mepivacaine good alternative
- Monitor vital signs
Liver disease:
- Caution with amides
- Consider reduced doses
- Articaine may be preferred
- Monitor for toxicity
Kidney disease:
- Metabolites accumulation risk
- Dose adjustment may be needed
- Careful monitoring required
Bleeding disorders:
- Avoid blocks with high hemorrhage risk
- Consider infiltration techniques
- Appropriate medical consultation
Allergy Considerations
True amide allergy (extremely rare):
- Consider ester alternatives
- Allergy testing referral
- Document carefully
- Have emergency protocols
Ester allergy:
- Use any amide anesthetic
- No cross-reactivity
- Avoid all esters including topicals
- Check for PABA sensitivity
Sulfite sensitivity:
- Avoid solutions with vasoconstrictors
- Use plain anesthetic solutions
- Check all product labels
Special Considerations and Contraindications
Absolute Contraindications
For all local anesthetics:
- Known allergy to specific agent
- Injection into infected tissue
- Uncontrolled medical conditions
For specific agents:
- Prilocaine in methemoglobinemia risk
- Cocaine with MAO inhibitors
- Articaine in children under 4 years
Relative Contraindications
Amides:
- Significant hepatic dysfunction
- Concurrent hepatotoxic drugs
- Severe cardiac conduction blocks
Esters:
- Atypical plasma cholinesterase
- Concurrent sulfonamide therapy
- G6PD deficiency (benzocaine)
Drug Interactions
Amides with:
- Cimetidine (reduced metabolism)
- Beta blockers (enhanced toxicity)
- CNS depressants (additive effects)
Esters with:
- Cholinesterase inhibitors
- Sulfonamides (PABA antagonism)
- Other ester-type drugs
Maximum Recommended Doses
With vasoconstrictor:
- Lidocaine: 7 mg/kg (max 500 mg)
- Mepivacaine: 6.6 mg/kg (max 400 mg)
- Articaine: 7 mg/kg (max 500 mg)
- Prilocaine: 8 mg/kg (max 600 mg)
Without vasoconstrictor:
- Lidocaine: 4.4 mg/kg (max 300 mg)
- Mepivacaine: 6.6 mg/kg (max 400 mg)
- Prilocaine: 8 mg/kg (max 600 mg)
Special Populations
Pediatric considerations:
- Calculate dose by weight
- Use lower concentrations
- Avoid overdose from multiple cartridges
- Consider psychological factors
Geriatric considerations:
- Reduced metabolic capacity
- Multiple drug interactions
- Cardiovascular sensitivity
- Careful dose calculation
Pregnancy and lactation:
- Use FDA Category B drugs
- Minimize first trimester exposure
- Consider fetal effects
- Document informed consent
Topical Anesthetic Selection
Benzocaine:
- Rapid onset
- Multiple concentrations available
- Methemoglobinemia risk
- Avoid in infants
Lidocaine:
- Available as gel, ointment, spray
- Good safety profile
- Slower onset than benzocaine
- Less risk of toxicity
Tetracaine:
- Long duration
- High toxicity potential
- Use sparingly
- Excellent effectiveness
EMLA (Eutectic Mixture):
- Lidocaine + prilocaine combination
- Excellent for intact skin
- Longer application time needed
- Useful in pediatrics
The classification of local anesthetics into amides and esters provides a fundamental framework for understanding their clinical behavior. Modern dentistry predominantly uses amide anesthetics due to their superior safety profile and rare allergic potential. However, understanding both classes remains essential for managing the occasional patient who cannot receive standard amide anesthetics and for utilizing the unique properties of specific agents to optimize patient care.